

The central atom, #"S"#, has three groups bonded to it, two oxygen atoms and a lone pair. #"AX"_2"E"# - two bond pairs and one lone pair The shape of the molecule is trigonal planar.Īll the atoms are in the same plane, and the #"F-B-F"# bond angles are all 120°. Minimizing the repulsion causes the #"F"# atoms to form an equilateral triangle about the #"B"# atom, as shown below. The #"B"# atom has three bond pairs in its outer shell.

The shape of the molecule is linear, and the #"Cl-Be-Cl"# bond angle is 180°. Repulsion between these two pairs causes the atoms to be as far apart as possible. The central #"Be"# atom has two bond pairs in its outer shell (SN = 2). The Lewis dot structure for #"BeCl"_2# is Lone pairs repel more than bond bonding pairs. You must determine the steric number (SN) - the number of bonding pairs and lone pairs about the central atom. The repulsion between valence electron pairs in the outer shell of the central atom determines the shape of the molecule. Use the VSEPR shape to determine the angles between the bonding pairs. Use the SN and VSEPR theory to determine the electron pair geometry of the molecule. That gives you the steric number (SN) - the number of bond pairs and lone pairs around the central atom. Write the Lewis dot structure of the molecule. doi: 10.1021/ed083p336.2.There are three basic steps to determining the molecular shape of a molecule: "Mechanisms that Interchange Axial and Equatorial Atoms in Fluxional processes: Illustration of the Berry Pseudorotation, the Turnstile and the Lever Mechanisms via animation of transition state normal vibrational modes". "Dynamic nuclear magnetic resonance study of fluorine exchange in liquid sulfur tetrafluoride". "The Trigonal Bipyramid Geometry (SN = 5) (VSEPR Part 4)" (PDF). The following compounds and ions have disphenoidal geometry: Sulfur tetrafluoride is the premier example of a molecule with the disphenoidal molecular geometry (see image at upper right). The four atoms in motion act as a lever about the central atom for example, the four fluorine atoms of sulfur tetrafluoride rotate around the sulfur atom. Thus, the 19F NMR spectrum of SF 4 (like that of PF 5) consists of single resonance near room temperature. This exchange of positions results in similar time-averaged environments for the two types of ligands. The ideal angle between the axial ligands and the equatorial ligands is 90° whereas the ideal angle between the two equatorial ligands themselves is 120°.ĭisphenoidal molecules, like trigonal bipyramidal ones, are subject to Berry pseudorotation in which the axial ligands move to equatorial positions and vice versa. Typically the bond distance to the axial ligands is longer than to the equatorial ligands.

The equatorial pair of ligands is situated in a plane orthogonal to the axis of the axial pair. The axial pair lie along a common bond axis so that are related by a bond angle of 180°. StructureĬompounds with disphenoidal geometry (See-Saw Geometry) have two types of ligands: axial and equatorial. Repulsion by bonding pairs at 120° is much smaller and less important. An equatorial lone pair is repelled by only two bonding pairs at 90°, whereas a hypothetical axial lone pair would be repelled by three bonding pairs at 90° which would make it stable.
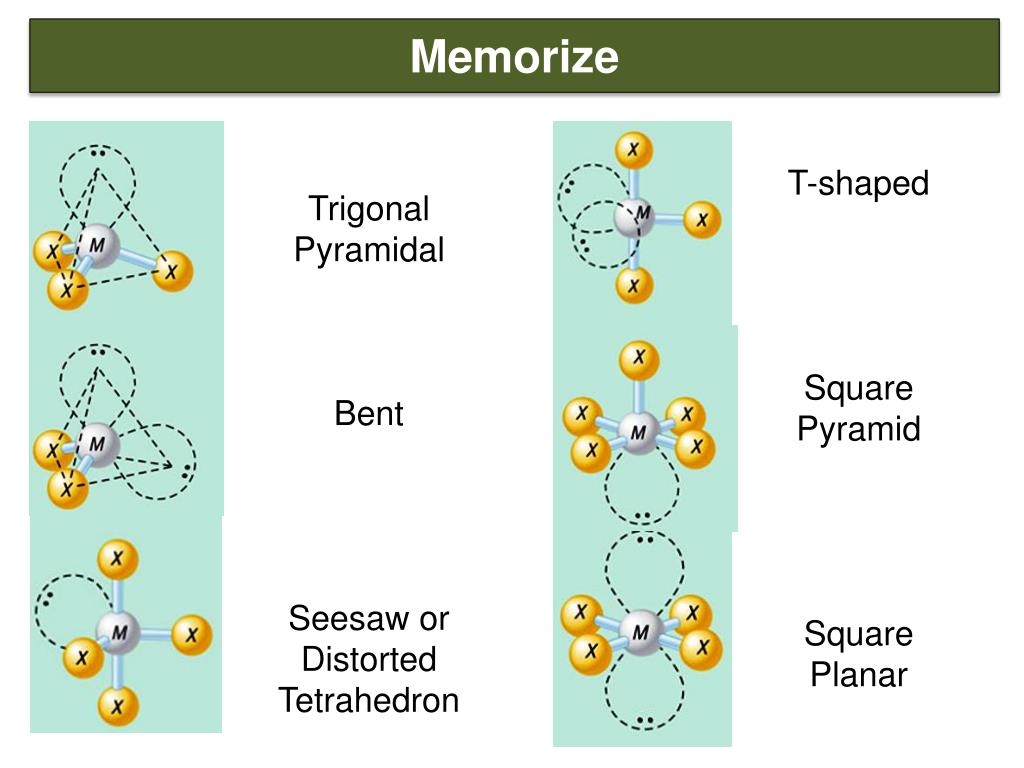
This is true because the lone pair occupies more space near the central atom (A) than does a bonding pair of electrons. An atom bonded to 5 other atoms (and no lone pairs) forms a trigonal bipyramid with two axial and three equatorial positions, but in the seesaw geometry one of the atoms is replaced by a lone pair of electrons, which is always in an equatorial position. The seesaw geometry occurs when a molecule has a steric number of 5, with the central atom being bonded to 4 other atoms and 1 lone pair (AX 4E 1 in AXE notation). Most commonly, four bonds to a central atom result in tetrahedral or, less commonly, square planar geometry. The name "seesaw" comes from the observation that it looks like a playground seesaw. Ideal ax-ax 180°, eq-eq 120°, ax-eq 90° SF 4 ax-ax 173.1°, eq-eq 101.6°ĭisphenoidal or seesaw (also known as sawhorse ) is a type of molecular geometry where there are four bonds to a central atom with overall C 2v molecular symmetry. Structural molecular geometry Seesaw molecular geometry


 0 kommentar(er)
0 kommentar(er)
